At the beginning of 2020, the new crown virus 2019-nCoV has spread globally. The World Health Organization announced on February 28 that the global risk level of the new crown pneumonia epidemic has been raised from the previous “high” to “very high”. According to the latest data released by WHO, as of 18:00 on March 31, Central European time (0:00 on April 1st, Beijing time), the total number of confirmed cases of new coronary pneumonia worldwide reached 754,948, and the cumulative number of deaths was 36,571. Countries where cases have been reported There are 202 in total and regions. The standard for the diagnosis of new coronary pneumonia is still a positive result of the new coronavirus nucleic acid test. The automated solution of ATMK® 2019-nCoV nucleic acid detection facilitates the nucleic acid detection of viruses; it greatly reduces manual operations during the processing of epidemic sample detection, optimizes the sample detection process, greatly shortens the time of viral nucleic acid detection, and improves the timeliness of diagnosis.
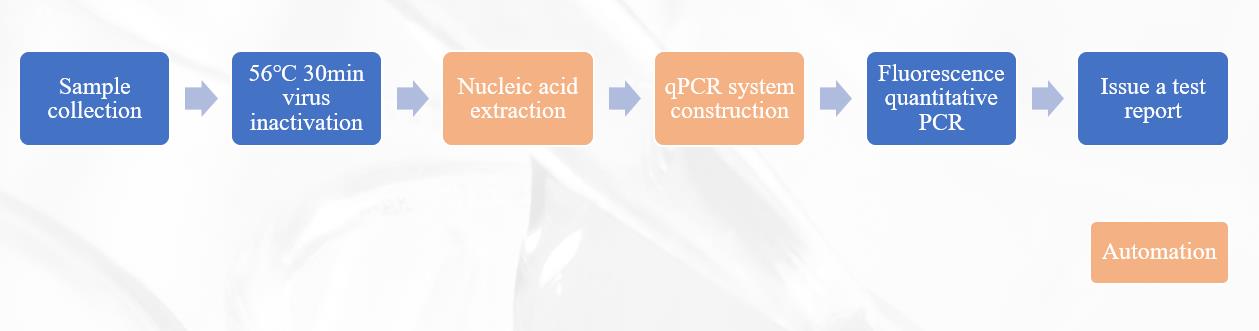
2019-nCoV Detection Flowchart
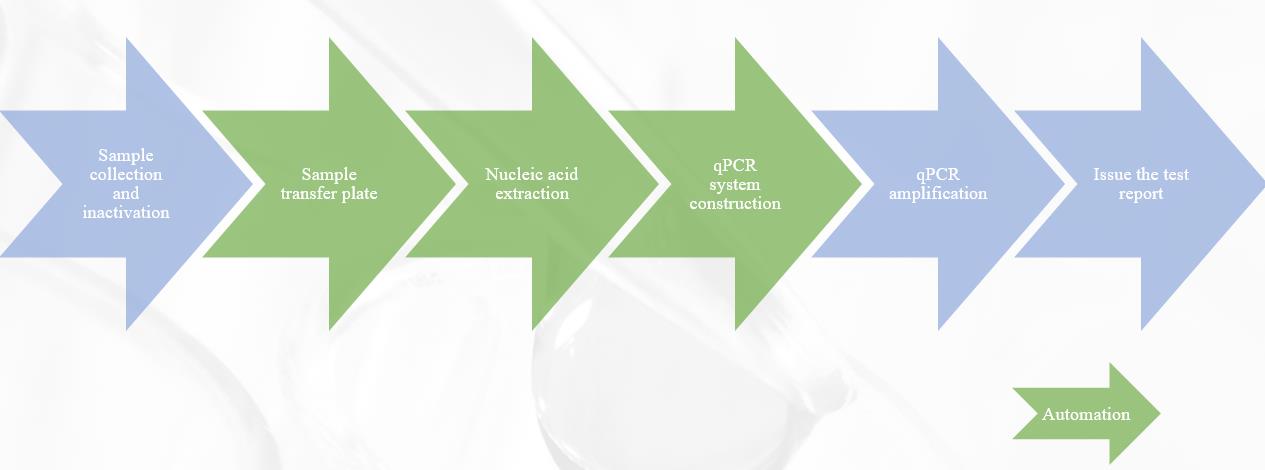
ATMK® 2019-nCoV automation solution
Solution advantages:
⬛ High degree of automation, reducing the risk of manual sample transfer
⬛ Flexible throughput, covering low, medium and high throughput samples
⬛ High-precision pipetting to better meet QPCR testing requirements
⬛Strong expansibility, can be matched with Kits from other manufacturers and QPCR detection kits