At present, NON-INVASIVE PRENATAL TESTING (NON-INVASIVE PRENATAL TESTING) is abbreviated as NIPT, which is already well known by ordinary consumers on the land of China. NIPT has experienced three stages of non-regulation, suspension, and supervision. Starting from November 2016, all medical institutions and medical laboratories with relevant qualifications can carry out non-invasive DNA prenatal screening and diagnosis. National policies have been continuously optimized and guided by the industry. Order development. In order to improve population quality, eugenics and education, and promote the development of medical and health care, local governments not only proposed NIPT free programs (such as Changsha, Shenzhen, etc.), but also clarified their testing fee standards to make them open and transparent, even in many provinces and cities. All are actively working to include the NIPT project in medical insurance, thereby reducing the cost of consumers. With the dividends brought about by the policy and the liberalization of the second-child policy, it is expected that the NIPT market will maintain a certain growth rate.
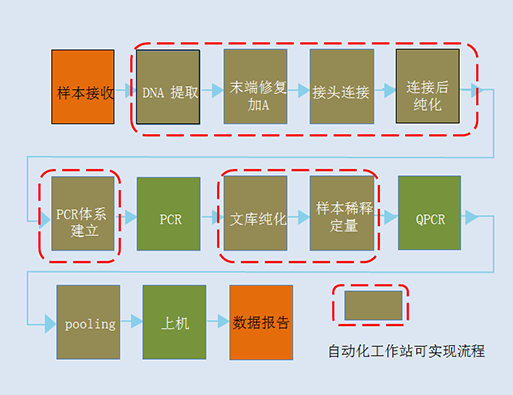
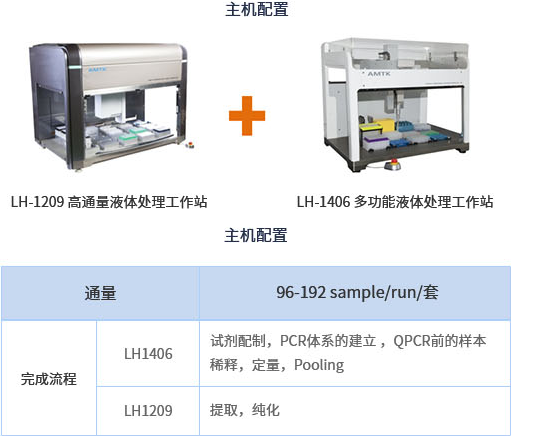
⬛ High throughput solution host configuration: this configuration can complete automatic NIPT detection of 96-192 samples

⬛ Medium flux scheme host configuration: this configuration can complete the automatic NIPT detection of 32-96 samples

⬛ Host configuration of low flux scheme: the scheme can complete automatic NIPT detection of 1-32 samples.

Comparison of Library concentration between 83 samples manual and amtk

The data shows that there is no significant difference in the concentration of QC between manual (QC control) and amtk workstation (QC test) libraries